Endocrine disruptors?
The most commonly accepted definition of endocrine disruptors is that proposed by the WHO in 2002:
"An endocrine disruptor is a substance or blend of substances, which alters the functions of the endocrine system. And thus induces harmful effects in an intact organism, in its offspring or in (sub)populations".

The mechanisms of action of endocrine disruptors
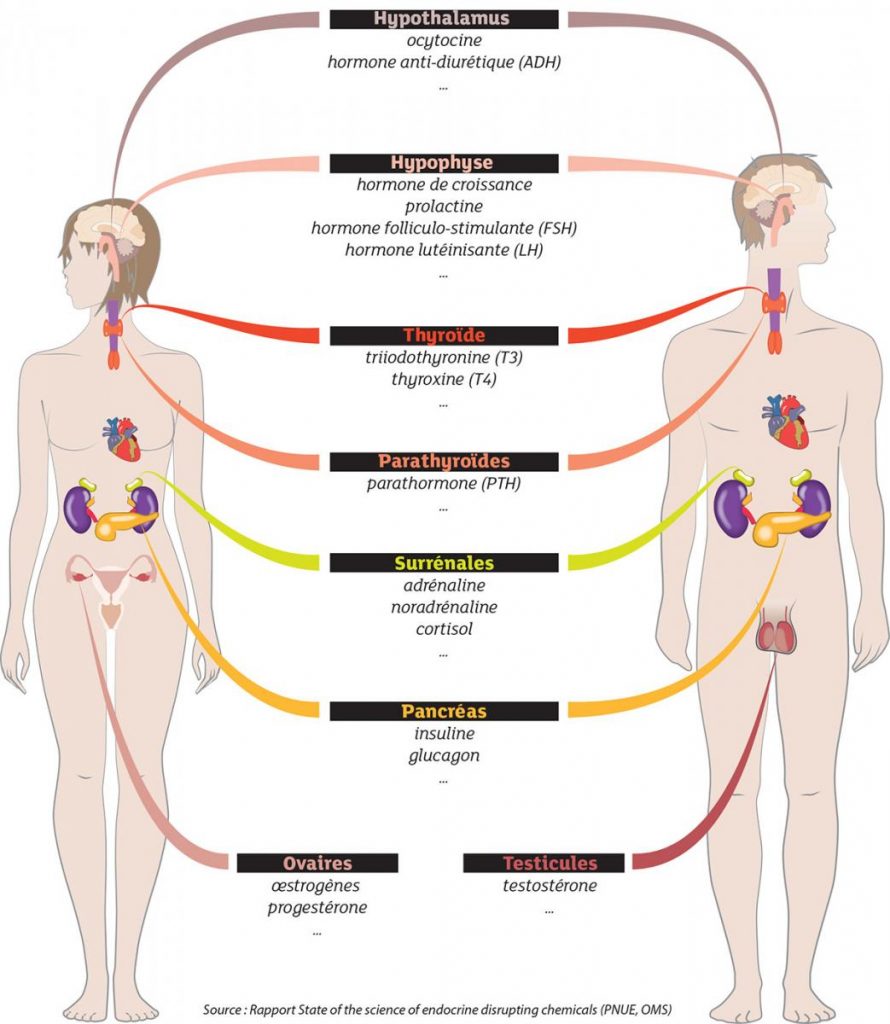
The endocrine system controls key body functions (growth, reproduction, metabolism, …) through hormones. Endocrine disruptors act by modifying the functioning of these hormones, which leads to pathological consequences that can be transgenerational.
The particularities of endocrine disruptors are:
The low level of exposure
Endocrine disruptors are suspected of acting like certain carcinogenic molecules, having an effect even at low doses.
Non-monotonic dose-response relationships
The harmful effects are not dependent on the absorbed dose and can be more important at lower doses than at higher doses.
Sensitivity varies according to the period of life
The effects can vary according to the period of life, with a more marked effect in particular during foeto-embryonic development, early childhood, or puberty.
‘Cocktail effects’
There may be synergies that increase the toxicity of mixed molecules compared to the effect they would have alone.
An evolving regulatory framework
Currently, 3 regulations legislate on endocrine disruptors:

The French government has detailed its actions on endocrine disruptors in the SNPE 2.
What about regulations? What will happen in 2022?
Article 13 of the AGEC law will come into force at the beginning of 2022. It aims to improve transparency for consumers by identifying endocrine disruptors when they are present in a product, based on a list of substances drawn up in collaboration with the ANSES.
European regulations will also integrate endocrine disruptors with, among others, the revision of the CLP regulation, scheduled for late 2022 – early 2023, which will integrate a new classification for endocrine disruptors.
SALVECO's ongoing policy on endocrine disruptors
In this complex technical and regulatory context, SALVECO’s strategy is in line with its DNA and values, with an approach that is always based on 4 strategic axes to be at the forefront of healthy alternatives.

I - Analytical approach
The first step of our strategy is to promptly identify substances or mixtures likely to have endocrine disrupting properties. The absence of such effects must be demonstrated on 4 axes: estrogenic, thyroid, androgenic, steroidogenesis.
In addition to our participation in the design of innovative analytical methods, we are also studying the interests and advantages of using a software screening method to evaluate our formulas. We have already performed preliminary tests which have not identified any endocrine activity for our formulas.
II - Collaborative approach
A collaborative approach allows us to support the implementation of tests to demonstrate the EP properties of a substance or a formula.
SALVECO is a member of the Pepper platform, a public-private research platform whose objective is to perform a pre-validation of test methods to characterize endocrine disrupting properties.


III - Scientific and technical analysis
Along with leading experts in the field, we have initiated experimental studies on our substances and formulations.
SALVECO has initiated discussions and collaborations with academic and industrial partners in order to better understand the complex mechanisms involved, as well as the link between the structure of the molecules and endocrine disruption.
IV - Demanding selection of our molecules
The health of the user is in the DNA of SALVECO. We use an extremely rigorous protocol for the choice of our raw materials, so that our formulas respect your health and the environment while maintaining product quality.
We maintain an active technological watch on existing lists of PE substances (proven, presumed and suspected) in order to cross-reference them with our catalog of molecules. If a molecule from our catalog appears in a PE list, it is withdrawn.

These 4 actions allow us to guarantee maximum safety when using our products.
Sources:
https://www.anses.fr/fr/content/travaux-et-implication-de-lanses-sur-les-perturbateurs-endocriniens
https://www.inserm.fr/dossier/perturbateurs-endocriniens/
